Learning Objectives
- Describe the structure of a water molecule.
- Describe the polarity of a water molecule.
A molecule of heavy water has two deuterium atoms in place of the two protium atoms of ordinary 'light' water. The weight of a heavy water molecule, however, is not substantially different from that of a normal water molecule, because about 89% of the molecular weight of water comes from the single oxygen atom. Hydrogen water is simply pure water with extra hydrogen molecules added to it. Hydrogen is a colorless, odorless, non-toxic gas that binds to other elements like oxygen, nitrogen, and carbon to. In a water molecule, the shared electrons are pulled closer to the oxygen atom than the hydrogen atoms, making the molecule dipolar-This makes water a good solvent for other ionic substances, which allows it to transport them -Water is also cohesive due its dipolar nature, which helps water to flow, allowing it to transport substances.
How much of the Earth’s surface is covered with water?
In his well-known poem “The Rime of the Ancient Mariner,” Samuel Coleridge wrote “Water, water everywhere, Nor any drop to drink.” The narrator was talking about being out on the ocean, but not having any water because he had killed an albatross (apparently bringing bad luck to everyone on the ship). About 75% of the Earth’s surface is water. The major constituent of the human body (over 60%) is water. This simple molecule plays important roles in all kinds of processes.

Structure of Water
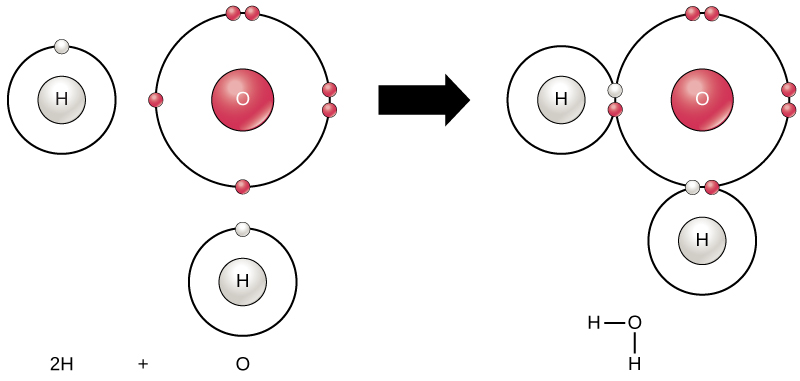
- The Configuration of the Water Molecule. A molecule of water is composed of two atoms of hydrogen and one atom of oxygen. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus.
- Also atom and molecules have fuzzy boundaries and the electron cloud of oxygen/water molecules will interact with that of the material around the hole, making passage difficult. If some or all holes are any larger, both molecules can get through. So a traditional approach of finding the membrane material may not work.
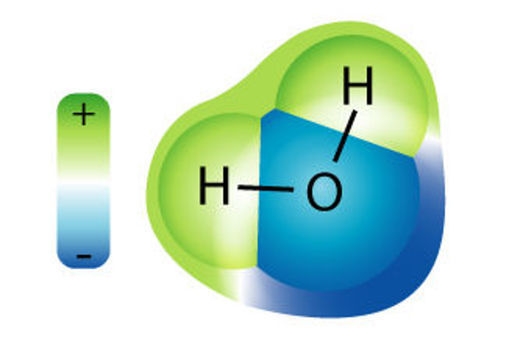
Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds). The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. As a result, the oxygen atom acquires a partial negative charge , while the hydrogen atoms each acquire a partial positive charge . The molecule adopts a bent structure because of the two lone pairs of electrons on the oxygen atom. The H-O-H bond angle is about 105°, slightly smaller than the ideal 109.5° of an sp3 hybridized atomic orbital.
The water molecule, visualized three different ways: ball-and-stick model, space-filling model, and structural formula with partial charges.
The bent shape of the water molecule is critical because the polar O-H bonds do not cancel one another and the molecule as a whole is polar. Figure 2 below illustrates the net polarity of the water molecule. The oxygen is the negative end of the molecule, while the area between the hydrogen atoms is the positive end of the molecule.
Figure 2. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom.
Polar molecules attract one another by dipole-dipole forces as the positive end of one molecule is attracted to the negative end of the nearby molecule. In the case of water, the highly polar O-H bonds results in very little electron density around the hydrogen atoms. Each hydrogen atom is strongly attracted to the lone-pair electrons on an adjacent oxygen atom. These are called hydrogen bonds and are stronger than conventional dipole-dipole forces.
Figure 3. A hydrogen bond is the attraction between a lone pair of electrons on the oxygen atom of one molecule and the electron-deficient hydrogen atom of a nearby molecule.
Because each oxygen atom has two lone pairs, it can make hydrogen bonds to the hydrogen atoms of two separate other molecules. The figure below shows the result – an approximately tetrahedral geometry around each oxygen atom consisting of two covalent bonds and two hydrogen bonds.
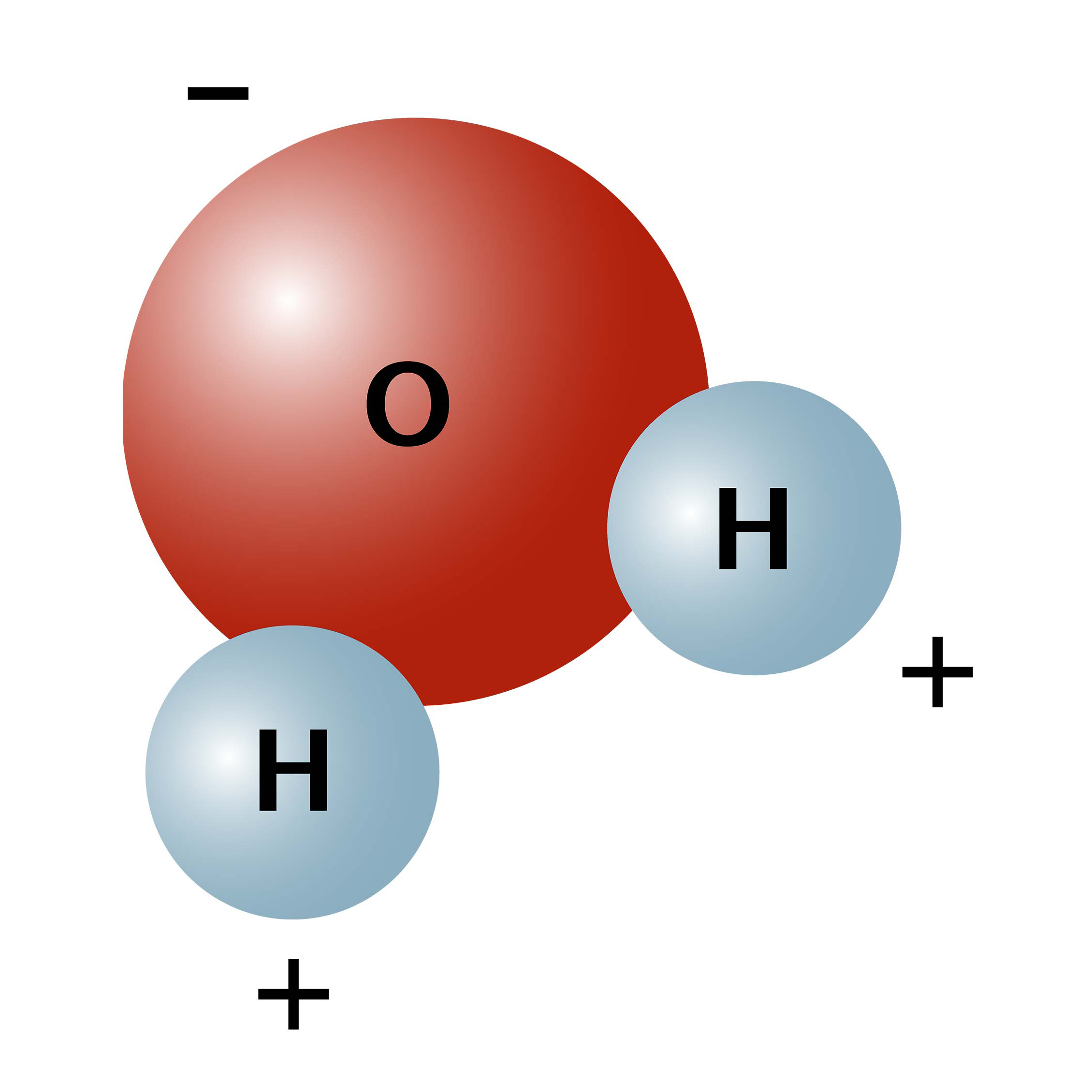
Figure 4. As a result of two covalent bonds and two hydrogen bonds, the geometry around each oxygen atom is approximately tetrahedral.
Summary
- Water is a molecular compound consisting of polar molecules that have a bent shape.
- The oxygen atom acquires a partial negative charge while the hydrogen atom acquires a partial positive charge.
Practice
Water Atomizer
Use the link below to answer the following questions:
Water Atomizer
- What is the H-O-H bond angle?
- How far is the center of each H atom from the center of the O atom?
Structure Of A Water Molecule
Review
- What type of bond exists in a water molecule?
- Which part of the molecule has a partial positive charge?
- Which part of the molecule as a partial negative charge?
- How do water molecules interact with one another?
Glossary
- electronegativity: A measure of the ability of an atom to attract the electrons when the atom is part of a compound.
- polar bond: A covalent bond in which electrons are shared unequally between two electrons.
